- Global
Get non-surgical solutions for today's top aesthetic concerns with Venus Treatments. Join thousands of satisfied patients worldwide!
- Loading...
- All Regions
Get non-surgical solutions for today's top aesthetic concerns with Venus Treatments. Join thousands of satisfied patients worldwide!
Achieve a fuller, natural-looking head of hair with minimally invasive hair restoration solutions.
Whether it’s thinning, receding, or just disappearing, hair loss is more than just a cosmetic issue—it can also have a significant impact on self-esteem, confidence, personal relationships, and even professional development. It’s estimated that by the age of 35, as many as two-thirds of American men will suffer from some degree of noticeable hair loss, and approximately 85% of American men experience thinning hair by the age of 501. It isn’t just men—women account for nearly half of all hair loss cases2, and many of them struggle even more with the emotional and psychological repercussions. If you’re one of them, now’s the time to take your life back.
Venus Concept’s minimally invasive hair restoration treatments help to restore a full, healthy head of hair without linear scarring, or the lengthy downtime associated with traditional hair transplant surgery. It works by using an advanced automated handpiece that extracts individual hair follicles from where they can be spared—usually on the back of the head above the nape of the neck—and then implants them in the areas of the scalp where you need them most. The thinning hair treatment works for all hair types, male or female, and leaves a natural-looking result you’ll love.
Venus Concept’s hair restoration procedures are the ideal solutions for male and female hair loss, using minimally invasive Follicular Unit Extraction (FUE) method for natural-looking results. Unlike traditional treatment for receding hairlines and thinning, FUE hair transplant does not require strips of the scalp to be surgically removed. Instead, individual hair follicles are extracted and a specialized handpiece then implants the grafts in areas of hair loss.
Both NeoGraft® treatments and ARTAS® hair loss solutions utilize the FUE method and relocate your own healthy hair follicles for a fast recovery process with no linear scarring. Traditional hair restoration treatment or follicle transplant procedures typically involve using a scalpel that greatly hinder recovery times. Whereas the FUE method paired with ARTAS®’s hair restoration solution or NeoGraft®’s advanced features can deliver visible results of a fuller, natural-looking hairline in 9 to 12 months.
Everything you need to know about your hair restoration treatment.
If you’re getting a hair restoration procedure done, your physician will be using the ARTAS® and/or NeoGraft® device.
Hair restoration procedures with ARTAS® and/or NeoGraft® are minimally invasive and designed to deliver excellent results. While most people can get the treatment, there are some factors that come into play to determine if you’re an ideal candidate. These include the number of grafts necessary to produce the desired results, the density and quality of the donor hair, hair texture, and potential for future hair loss. Your treatment provider will be able to help to determine if you are a suitable candidate for a hair restoration procedure.
The NeoGraft® hair restoration procedure is minimally invasive, and a viable option for both men and women looking to restore their own living and growing hair with transplantation.
Traditional hair restoration procedures usually employ a technique known as the strip method, which involves surgically removing a strip from the patient’s scalp (the back of the head) to be used as the donor site. This leaves a permanent linear scar. Follicular Unit Extraction on the other hand, a method used by both ARTAS® and NeoGraft®, harvests individual hair follicles from areas that consist of terminal hair, which are naturally resistant to hair loss. It then implants those grafts at the site of hair loss, where, over time, they will regrow as healthy, fully functioning hair. As a result, this particular hair restoration procedure does not leave a linear scar, is more comfortable, and requires much less recovery time.
FUE stands for Follicular Unit Extraction. It is an advanced, minimally invasive hair transplant method that allows for the harvesting of individual follicles from the back of the head (donor area) without a scalpel or stitches, and therefore leaves no linear scar.
The physician will be highly involved in making all of the necessary and important decisions, and will be there every step of the way. Certified hair restoration technicians will be the ones who are assisting the physician during the procedure. These technicians all come with more than five years of experience and are recertified annually.
While pricing may be similar, the fee structure for ARTAS® and/or NeoGraft® hair transplant procedures will reflect the more detailed and intricate nature of the procedure, compared to those performed with the strip method.
For the procedure, the donor area will need to be shaved down with a zero guard in the harvesting area. As such, your treatment provider may advise you to cut your hair in preparation for shaving of the donor site at the start of your procedure. In addition to making this part of the process easier, cutting the hair short will also make for a less noticeable change after you return to your daily routine while the donor site heals and covers with new hair. For patients with longer hair, the donor area can be shaved in a way that it can be disguised by keeping the hair around it long to hide the shaved areas.
No. ARTAS® and/or NeoGraft® hair restoration procedures can be done by just shaving the needed donor area at the back of your scalp.
Depending on the number of grafts, the treatment can take anywhere from 4-10 hours. The number of treatments will depend on your unique circumstances, but in many cases, just one procedure can be enough to attain desirable results.
Your physician will numb the area with a local anesthesia before beginning the procedure, so you will likely feel minimal discomfort while the hair transplant is being done. Some physicians also offer the option of mild sedation for the duration of the procedure, depending on your preference. Once the numbing wears off and as the grafts heal, you may experience some tenderness and minor swelling post-procedure around the donor site. This is a normal part of the healing process and should subside in a few days. Always be sure to consult with your physician with regards to post-procedure care and pain management.
No result can ever be guaranteed, but the before-and-after photos from previous ARTAS® and/or NeoGraft® patients show that incredible results are very likely. With our certified treatment providers, you can rest assured that you are in very good hands. Your physician will set as realistic expectations as possible and make sure all your questions are answered. For more real patient results, go to RealSelf.com, where ARTAS® and/or NeoGraft® hair transplants carry an average 95% or higher“Worth It” rating from past patients.
There's typically very little pain or discomfort after the procedure. You may experience a slight amount of drainage for the first day after your procedure, but this is minimal. You must refrain from strenuous activity for two weeks, to ensure you do not cause any harm to the newly implanted grafts. You can expect the newly implanted grafts to be slightly raised in the first week after the procedure. Around three to four days later, they will start to scab over and then possibly shed. Shedding is good and a natural part of the healing process.
Recovery time after a FUE hair restoration procedure like ARTAS® and/or NeoGraft® is much faster than traditional hair transplants, because there is no incision involved. The newly implanted grafts may feel tender and appear slightly raised at first, but this should subside in one week. You may also see some scabbing, but this should also flake off within a week. Your physician will instruct you on how to properly care for your new grafts, but most patients are able to return to their usual routine within two weeks after completing the procedure.
Immediately after your treatment, the physician will ensure that the new grafts are secure and in their proper position. They will thoroughly rinse the treated areas and a light protective dressing may be applied. You will be given detailed instructions about how to care for the grafts when you go home, including any recommended shampoos, topical antiseptics, or hair care products. Because there is no incision, there is no need to get any sutures removed.
Extraordinary results speak for themselves
Improve the appearance of thinning hair on the crown, or the upper back area of the head, which is where many men see the first signs of hair loss.
Devices that offer hair restoration: NeoGraft®, ARTAS®
Results after: 1 treatment
Graft Count: 800
Courtesy of Ohio Facial Plastics, Sumit Bapna, MD
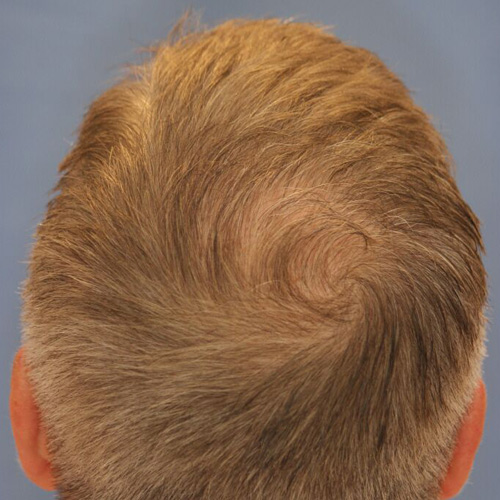 After
After
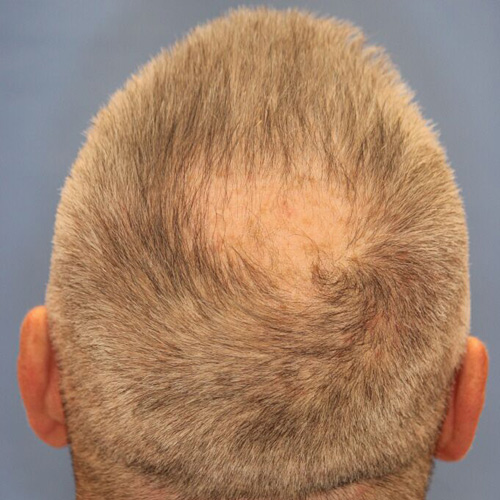 Before
BeforeCorrect a receding hairline by adding more fullness on the top of the head and around the temples.
Devices that offer hair restoration: NeoGraft®, ARTAS®
Results after: 2 treatments
Graft Count: 4,110
Courtesy of Blackhawk Plastic Surgery, Stephen J. Ronan, MD
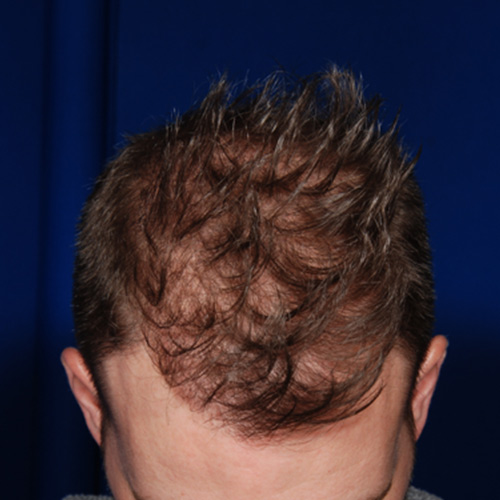 After
After
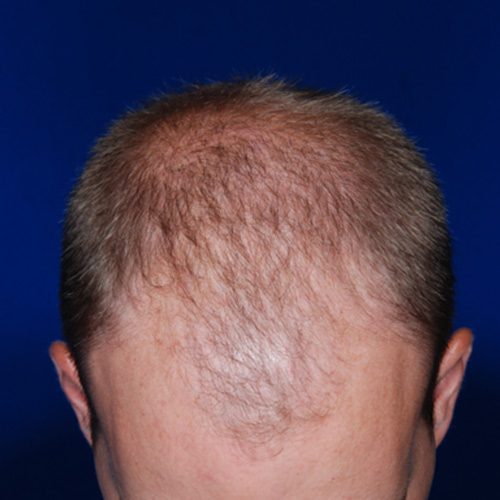 Before
BeforeCorrect a receding hairline by adding more fullness on the top of the head and around the temples.
Devices that offer hair restoration: NeoGraft®, ARTAS®
Results after: 1 treatment
Graft Count: 2,600
Courtesy of Paramount Plastic Surgery, Keith Jeffords, MD
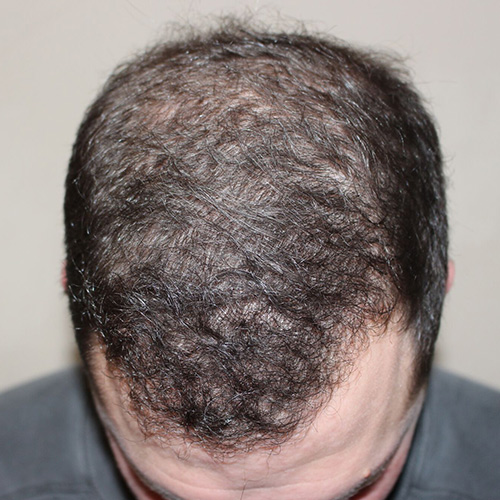 After
After
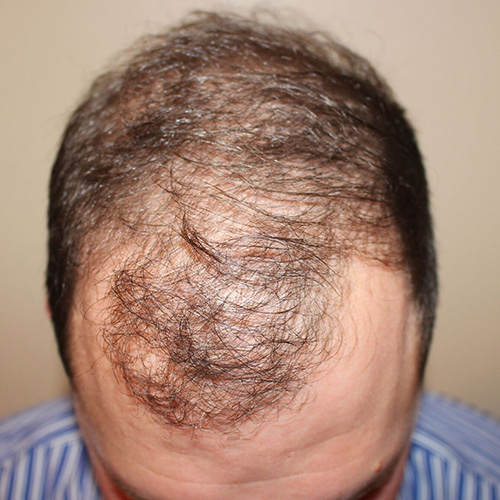 Before
BeforeAddress thinning on the top and front of the scalp—the most common problem areas for women—by restoring thicker, fuller, and healthier-looking hair.
Devices that offer hair restoration: NeoGraft®
Results after: 1 treatment
Graft Count: 1,200
Courtesy of H/K/B Cosmetic Surgery, Joseph Hunstad, MD; Bill Kortesis, MD; & Gaurav Bharti, MD
 After
After
 Before
BeforeImprove thinning on the front of the scalp, as well as a receding hairline around the temples.
Devices that offer hair restoration: NeoGraft®, ARTAS®
Norwood Stage: 5
Results documented: 12 months after ARTAS®
Graft count: 1886 grafts
Courtesy of Bernstein Medical – Center For Hair Restoration, Robert M. Bernstein, MD
 After
After
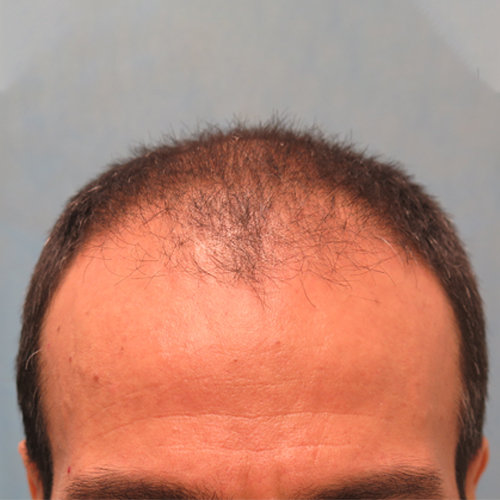 Before
BeforeImprove thinning on the front of the scalp, as well as a receding hairline around the temples.
Devices that offer hair restoration: NeoGraft®, ARTAS®
Norwood Stage: 5
Results documented: 12 months after ARTAS®
Graft count: 1886 grafts
Courtesy of Bernstein Medical – Center For Hair Restoration, Robert M. Bernstein, MD
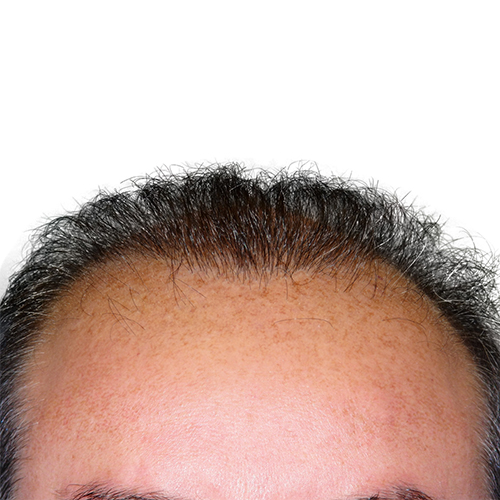 After
After
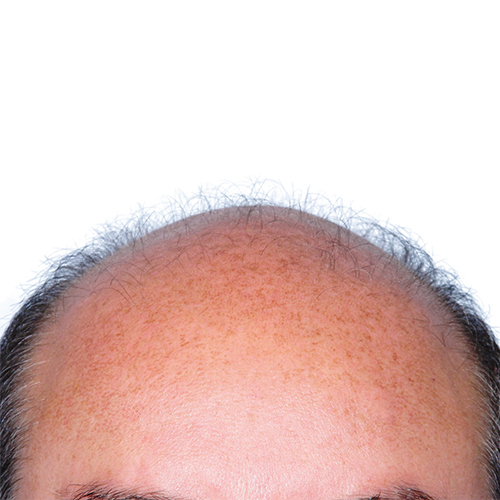 Before
BeforeImprove thinning on the front of the scalp, as well as a receding hairline.
Devices that offer hair restoration: NeoGraft®, ARTAS®
Norwood Stage: 3A
Results documented: 9 months after ARTAS®
Graft count: 1066 grafts
Courtesy of the Hair Sciences Center of Colorado, James A.Harris, MD, FACS
 After
After
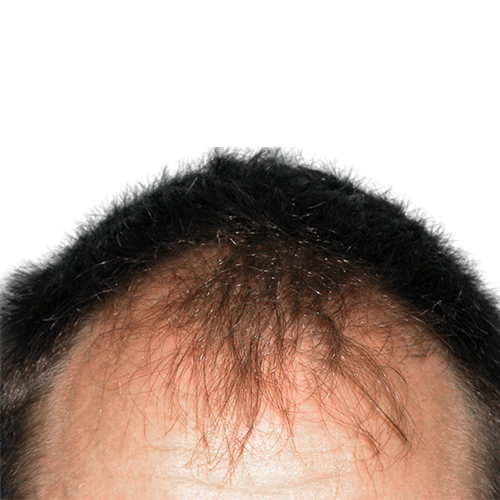 Before
BeforeINDICATIONS FOR USE:
NeoGraft® is cleared by the FDA, licensed by Health Canada and has CE Mark with indication for use in suction-assisted follicular extraction and re-implantation. It is an auto-graft system and can be used on both male and female patients.
ARTAS® iX is cleared by the FDA, licensed by Health Canada and has CE Mark with indication for use for harvesting hair follicles from the scalp in men diagnosed with androgenic alopecia (male pattern hair loss) who have black or brown straight hair. ARTAS® iX is intended to assist physicians in identifying and extracting hair follicular units from the scalp during hair transplantation; creating recipient sites; and implanting harvested hair follicles.
ARTAS® is cleared by the FDA, licensed by Health Canada and has CE Mark with indication for use for harvesting hair follicles from the scalp in men diagnosed with androgenic alopecia (male pattern hair loss) who have black or brown straight hair. It is also indicated for creating recipient sites for subsequent manual implantation of harvested follicles.
REFERENCES:
1. American Hair Loss Association. Men’s Hair Loss: Introduction. (n.d.). Retrieved from https://www.americanhairloss.org/men_hair_loss/introduction.html↩
2. U.S. National Library of Medicine. Androgenetic alopecia. (n.d.). Retrieved from https://ghr.nlm.nih.gov/condition/androgenetic-alopecia#statistics↩
Click the button below to be directed to the dedicated NeoGraft® website, where you can search for a certified hair restoration provider in your area.
For more information call: (888) 907-0115 // [email protected] // 235 Yorkland Blvd., Suite 900, Toronto, ON, M2J 4Y8 Canada
REGULATORY CLEARANCES [ More ]
Venus Bliss™ is cleared by the FDA and licensed by Health Canada for non-invasive lipolysis of the abdomen and flanks in individuals with a Body Mass Index (BMI) of 30 or less, with the diode laser applicators. The (MP)2 applicator is cleared by the FDA for temporary reduction in the appearance of cellulite, and licensed by Health Canada for temporary increase of skin tightening, temporary circumferential reduction, and temporary cellulite reduction. Venus Bliss™ has CE Mark as a non-invasive medical aesthetic device enabling a comprehensive approach leading to body contouring, addressing fat reduction, skin tightening, circumference reduction, and cellulite reduction.
Venus Versa™ is cleared by the FDA, licensed by Health Canada, and has CE Mark as a multi-application device intended to be used in aesthetic and cosmetic procedures. The SR515 and SR580 applicators are cleared by the FDA, licensed by Health Canada, and have CE Mark for the treatment of benign pigmented epidermal and cutaneous lesions and treatment of benign cutaneous vascular lesions. The HR650/HR650XL and HR690/HR690XL applicators are cleared by the FDA, licensed by Health Canada, and have CE Mark for the removal of unwanted hair and to effect stable long-term or permanent hair reduction for Fitzpatrick skin types I-IV. The AC Dual applicator is cleared by the FDA, licensed by Health Canada, and has CE Mark for the treatment of acne vulgaris. The DiamondPolar™ and OctiPolar™ applicators on the Venus Versa™ system are cleared by the FDA for non-invasive treatment of moderate to severe facial wrinkles and rhytides on females with Fitzpatrick skin types I-IV. The DiamondPolar™ applicator is licensed by Health Canada and has CE Mark for non-invasive treatment of moderate to severe facial wrinkles and rhytides on females with Fitzpatrick skin types I-IV. The OctiPolar™ applicator on the Venus Versa™ system is licensed by Health Canada and has CE Mark for temporary body contouring via skin tightening, circumferential reduction, and cellulite reduction. The NanoFractional RF™ (Viva) applicator is cleared by the FDA, licensed by Health Canada, and has CE Mark for dermatological procedures requiring ablation and resurfacing of the skin.
NeoGraft® is cleared by the FDA, licensed by Health Canada and has CE Mark with indication for use in suction-assisted follicular extraction and re-implantation. It is an auto-graft system and can be used on both male and female patients.
ARTAS iX™ is cleared by the FDA, licensed by Health Canada and has CE Mark with indication for use for harvesting hair follicles from the scalp in men diagnosed with androgenic alopecia (male pattern hair loss) who have black or brown straight hair. ARTAS iX™ is intended to assist physicians in identifying and extracting hair follicular units from the scalp during hair transplantation; creating recipient sites; and implanting harvested hair follicles.
Venus Legacy™ is cleared by the FDA for the non-invasive treatment of moderate to severe facial wrinkles and rhytides in females with Fitzpatrick skin types I-IV with the OctiPolar™ and DiamondPolar™ applicators, and temporary reduction in the appearance of cellulite with the 4D Body (LB2) and 4D Face (LF2) applicators. It is licensed by Health Canada and has CE Mark for the temporary increase of skin tightening, temporary circumferential reduction, temporary cellulite reduction, and temporary wrinkle reduction.
Venus Velocity™ is cleared by the FDA, licensed by Health Canada and has CE Mark for hair removal, permanent hair reduction (defined as the long-term stable reduction in the number of hairs re-growing when measured at 6, 9 and 12 months after the completion of a treatment regimen), and the treatment of pseudofolliculitis barbae for all Fitzpatrick skin types.
Venus Fiore™ received regulatory approval in Israel for aesthetic and functional treatment of the vagina, labia and mons pubis. Venus Fiore™ is available for sale in India, Hong Kong, and other selected Asian countries.
Venus Viva™ is cleared by the FDA, licensed by Health Canada, and has CE Mark for dermatological procedures requiring ablation and resurfacing of the skin. The DiamondPolar™ applicator is cleared by the FDA, licensed by Health Canada and has CE Mark for the treatment of moderate to severe wrinkles and rhytides in Fitzpatrick skin types I-IV.
Venus Freeze Plus™ is cleared by the FDA for the non-invasive treatment of moderate to severe facial wrinkles and rhytides in females with Fitzpatrick skin types I-IV. It is licensed by Health Canada for temporary skin tightening, and temporary reduction in the appearance of cellulite on the abdomen and flanks, using the DiamondPolar™ and OctiPolar™ applicators. The DiamondPolar™ applicator on Venus Freeze Plus™ has CE Mark for the non-invasive treatment of moderate to severe facial wrinkles and rhytides, and the increase of skin tightening, temporary circumferential reduction, and cellulite reduction with the OctiPolar™ applicator.
Venus Heal™ is licensed by Health Canada and can be used for the treatment of both acute and chronic disorders of the musculoskeletal system, such as muscle spasms, back pain, and soft tissue injuries, and results in effects such as pain relief, myorelaxation, increase of local blood circulation, and edema reduction. In the U.S., Venus Heal™ is cleared by the FDA for the relief of minor muscle aches and pain, relief of muscle spasm, and temporary improvement of local blood circulation. These indications enable the treatment of certain soft tissue injuries and conditions.
Venus Glow™ is cleared by the FDA as a Class I motorized dermabrasion device. It provides a dermal rejuvenation treatment that works to open up and deep-clean pores. Venus Concept is the exclusive distributor for Venus Glow™.
Venus Epileve™ is licensed by Health Canada and has CE Mark for hair removal, permanent hair reduction (defined as the long-term stable reduction in the number of hairs re-growing when measured at 6, 9 and 12 months after the completion of a treatment regimen), and the treatment of pseudofolliculitis barbae for all Fitzpatrick skin types. Venus Epileve™ is also CE-Marked for hirsutism.
Venus Freeze™ is cleared by the FDA for the non-invasive treatment of moderate to severe facial wrinkles and rhytides in females with Fitzpatrick skin types I-IV. It is licensed by Health Canada for temporary skin tightening, and temporary reduction in the appearance of cellulite on the abdomen and flanks, using the DiamondPolar™ and OctiPolar™ applicators. The DiamondPolar™ applicator on Venus Freeze™ has CE Mark for the non-invasive treatment of moderate to severe facial wrinkles and rhytides, and the increase of skin tightening, temporary circumferential reduction, and cellulite reduction with the OctiPolar™ applicator.
Venus Swan™ is cleared by the FDA for the non-invasive treatment of moderate to severe facial wrinkles and rhytides, and licensed by Health Canada for the non-invasive treatment of cellulite reduction, skin tightening, and temporary reduction in the appearance of stretch marks.
Copyright © 2025 Venus Concept. All rights reserved.
You are entering our website. For other countries/regions and language options, please click the SELECT A DIFFERENT REGION button below.
SELECT A DIFFERENT REGIONAre you looking to get a treatment? Please visit our patient website to learn more.
Click HereUnsure which aesthetic treatment is right for you? Take this quick and easy quiz to discover treatments that suit your needs.
Get Started