- Global
Get non-surgical solutions for today's top aesthetic concerns with Venus Treatments. Join thousands of satisfied patients worldwide!
- Loading...
- All Regions
Get non-surgical solutions for today's top aesthetic concerns with Venus Treatments. Join thousands of satisfied patients worldwide!
Focus on reaching your body goals, your way! Venus Bliss™ body fat reduction provides safe, comfortable, non-surgical treatments for body fat reduction around the abdomen and flanks to help you better achieve your body goals. Learn why Venus Bliss™ is right for you!
Everybody has their own reasons for wanting to make a change, whether it’s to enhance your workout results, trim down for a special occasion, or just feel more confident in your own skin again. It’s no wonder why belly fat reduction was the most in-demand non-surgical treatment for both men and women in 2018, after injectables—it actually had the highest representation of male patients compared to any other non-surgical procedure.1
While there’s certainly no one-size-fits-all when it comes to reaching your body goals, Venus Bliss™ can help. Using diode laser fat reduction and complementary radio frequency technology, these treatments work to tighten skin*, smooth cellulite, and further reduce circumference* without surgery or lengthy recovery periods. Now you can enjoy comfortable fat treatments with natural results.

Tennis legend, entrepreneur, fashion icon, and women’s rights advocate, Venus Williams has taken on the role as our Venus Bliss™ global celebrity brand ambassador. See how your business can leverage this campaign.
Fat treatments with Venus Bliss™ are powered by diode laser technology within the laser lipolysis machine. Using up to four applicators that are applied on the abdomen and/or flanks, energy penetrates deep below the skin’s surface where the fat is heated and broken down.
The Venus Bliss™ device also offers skin tightening* and cellulite reduction treatments use a proprietary combination of energies to boost collagen and elastin fibers—essential components for strengthening the connective tissue that keeps lax skin and cellulite at bay.
![]() CLINICALLY PROVEN RESULTS:
CLINICALLY PROVEN RESULTS:
One study showed that 75% of the patients were satisfied with the results of their Venus Bliss™ treatments and would recommend it to a friend.2
![]() COMFORTABLE TREATMENT EXPERIENCE:
COMFORTABLE TREATMENT EXPERIENCE:
As many as 90% of patients in the study reported that they found the diode laser fat reduction treatment and the Venus Bliss™ machine to be comfortable.3
![]() SAFE FOR ALL SKIN TONES:
SAFE FOR ALL SKIN TONES:
Technologies used in Venus Bliss™ are all proven to be safe and effective for all skin tones, including even darker complexions.
![]() NO DOWNTIME:
NO DOWNTIME:
Return to your daily routine immediately after completing your treatment session.
Everything you need to know about your Venus Bliss™ treatment
Venus Bliss™ treatments are safe for patients of any skin tone with a Body Mass Index of 30 or less. However, for safety reasons, you may not be able to get diode laser fat reduction treatment if you have/are:
Yes. Venus Bliss™ is safe for all skin tones, even darker ones.
A single Venus Bliss™ treatment typically takes 25 minutes to complete.
Every patient is unique; however, a Venus Bliss™ treatment may be effective with just one treatment. Optimal results will be seen three to six months after completing your treatment plan.
Venus Bliss™ treatments should be performed six weeks apart.
Just make sure your skin is clean. Do not apply any lotions or creams on the area you’re having treated immediately before your session. Also note that the treatment cannot be done over tattoos. Before starting, your treatment provider will likely take a “before” photo, check your weight, examine the area for any irregularities, and mark out the treatment area with a white pencil.
Each Venus Bliss™ treatment lasts 25 minutes.
A Venus Bliss™ laser fat reduction treatment will start with a cooling phase for a few seconds where you’ll feel a slight chill from the applicators on your skin. You will then gradually feel the skin getting warmer. Once the temperature builds, the applicators will switch to cooling mode again for a brief period to maintain the therapeutic temperature in the fat layer and to keep the treatment as comfortable as possible. This process of heating and cooling will continue for the duration of your treatment.
A Venus Bliss™ treatment shouldn’t be painful. You may feel a bit of discomfort as the applicators get warmer—this is expected as part of the non-invasive process for targeting the underlying fat—but it should still be manageable
The area may look a little flushed and feel tender, and some patients may experience a little bit of swelling, firmness, or bruising. All of these are normal and common following a Venus Bliss™ treatment. The tenderness may take up to two weeks to resolve but it should be mild enough to not interfere with your daily activities. Of course, if something doesn’t feel quite right, always consult with your treatment provider.
There is no downtime and no physical restrictions that you need to worry about. You should be able to resume your regular daily activities immediately after completing a Venus Bliss™ treatment.
Your treatment provider may recommend massaging the treatment area for five to 10 minutes, one to two times a day. Otherwise you don’t need to do anything after your Venus Bliss™ diode laser fat reduction treatment.
Extraordinary results speak for themselves
Treatments work to break down and destroy fat cells in the abdomen, resulting in a smoother, flatter midsection.
Results after: 1 treatment
Courtesy of Suzanne Kilmer, MD
 After
After
 Before
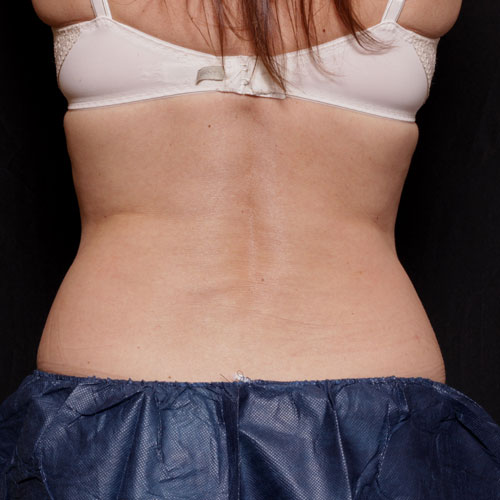
BeforeTreating the flanks will help to give the body a more contoured shape, which in turn can help you look leaner from all angles.
Results after: 1 treatment
Courtesy of Suzanne Kilmer, MD
 After
After
 Before
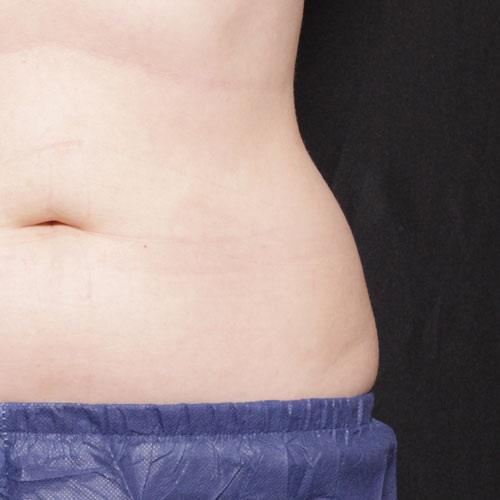
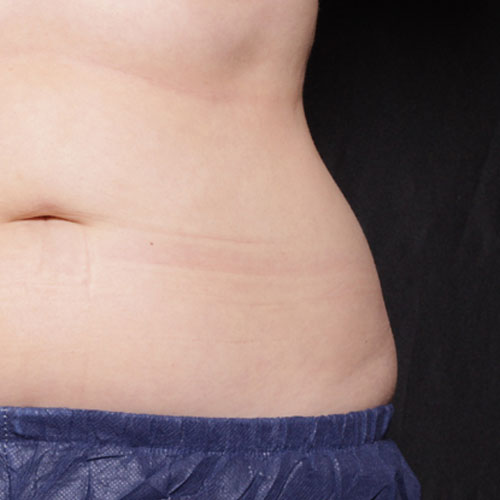
BeforeTreatments on the abdomen and/or flanks work to reduce focal fat, giving your midsection a narrower circumference and more contoured silhouette.
Results after:1 treatment
Courtesy of Suzanne Kilmer, MD
 After
After
 Before
BeforeINDICATIONS FOR USE:
*Venus Bliss™ is cleared by the FDA and licensed by Health Canada for non-invasive lipolysis of the abdomen and flanks in individuals with a Body Mass Index (BMI) of 30 or less, with the diode laser applicators. The (MP)2 applicator is cleared by the FDA for temporary reduction in the appearance of cellulite, and licensed by Health Canada for temporary increase of skin tightening, temporary circumferential reduction, and temporary cellulite reduction. Venus Bliss™ has CE Mark as a non-invasive medical aesthetic device enabling a comprehensive approach leading to body contouring, addressing fat reduction, skin tightening, circumference reduction, and cellulite reduction.↩
*Venus Bliss™ is cleared by the FDA and licensed by Health Canada for non-invasive lipolysis of the abdomen and flanks in individuals with a Body Mass Index (BMI) of 30 or less, with the diode laser applicators. The (MP)2 applicator is cleared by the FDA for temporary reduction in the appearance of cellulite, and licensed by Health Canada for temporary increase of skin tightening, temporary circumferential reduction, and temporary cellulite reduction. Venus Bliss™ has CE Mark as a non-invasive medical aesthetic device enabling a comprehensive approach leading to body contouring, addressing fat reduction, skin tightening, circumference reduction, and cellulite reduction.↩
*Venus Bliss™ is cleared by the FDA and licensed by Health Canada for non-invasive lipolysis of the abdomen and flanks in individuals with a Body Mass Index (BMI) of 30 or less, with the diode laser applicators. The (MP)2 applicator is cleared by the FDA for temporary reduction in the appearance of cellulite, and licensed by Health Canada for temporary increase of skin tightening, temporary circumferential reduction, and temporary cellulite reduction. Venus Bliss™ has CE Mark as a non-invasive medical aesthetic device enabling a comprehensive approach leading to body contouring, addressing fat reduction, skin tightening, circumference reduction, and cellulite reduction.↩
REFERENCES:
1. American Society for Aesthetic Plastic Surgery. (2018). 2018 Cosmetic (Aesthetic) Surgery National Data Bank Statistics. Retrieved from https://www.surgery.org/sites/default/files/ASAPS-Stats2018_0.pdf↩
2. Data on file↩
3. Ibid↩
Search below to find a provider near you and to learn about our non-surgical aesthetic treatments with ARTAS®, NeoGraft®, Venus Bliss™, Venus Blilss MAX™, Venus Versa™, Venus Legacy™ Venus Versa™ Pro, Venus Velocity™, Venus Viva™ MD, and Venus Glow™.
For more information call: (888) 907-0115 // [email protected] // 235 Yorkland Blvd., Suite 900, Toronto, ON, M2J 4Y8 Canada
REGULATORY CLEARANCES [ More ]
Venus Bliss™ is cleared by the FDA and licensed by Health Canada for non-invasive lipolysis of the abdomen and flanks in individuals with a Body Mass Index (BMI) of 30 or less, with the diode laser applicators. The (MP)2 applicator is cleared by the FDA for temporary reduction in the appearance of cellulite, and licensed by Health Canada for temporary increase of skin tightening, temporary circumferential reduction, and temporary cellulite reduction. Venus Bliss™ has CE Mark as a non-invasive medical aesthetic device enabling a comprehensive approach leading to body contouring, addressing fat reduction, skin tightening, circumference reduction, and cellulite reduction.
Venus Versa™ is cleared by the FDA, licensed by Health Canada, and has CE Mark as a multi-application device intended to be used in aesthetic and cosmetic procedures. The SR515 and SR580 applicators are cleared by the FDA, licensed by Health Canada, and have CE Mark for the treatment of benign pigmented epidermal and cutaneous lesions and treatment of benign cutaneous vascular lesions. The HR650/HR650XL and HR690/HR690XL applicators are cleared by the FDA, licensed by Health Canada, and have CE Mark for the removal of unwanted hair and to effect stable long-term or permanent hair reduction for Fitzpatrick skin types I-IV. The AC Dual applicator is cleared by the FDA, licensed by Health Canada, and has CE Mark for the treatment of acne vulgaris. The DiamondPolar™ and OctiPolar™ applicators on the Venus Versa™ system are cleared by the FDA for non-invasive treatment of moderate to severe facial wrinkles and rhytides on females with Fitzpatrick skin types I-IV. The DiamondPolar™ applicator is licensed by Health Canada and has CE Mark for non-invasive treatment of moderate to severe facial wrinkles and rhytides on females with Fitzpatrick skin types I-IV. The OctiPolar™ applicator on the Venus Versa™ system is licensed by Health Canada and has CE Mark for temporary body contouring via skin tightening, circumferential reduction, and cellulite reduction. The NanoFractional RF™ (Viva) applicator is cleared by the FDA, licensed by Health Canada, and has CE Mark for dermatological procedures requiring ablation and resurfacing of the skin.
NeoGraft® is cleared by the FDA, licensed by Health Canada and has CE Mark with indication for use in suction-assisted follicular extraction and re-implantation. It is an auto-graft system and can be used on both male and female patients.
ARTAS iX™ is cleared by the FDA, licensed by Health Canada and has CE Mark with indication for use for harvesting hair follicles from the scalp in men diagnosed with androgenic alopecia (male pattern hair loss) who have black or brown straight hair. ARTAS iX™ is intended to assist physicians in identifying and extracting hair follicular units from the scalp during hair transplantation; creating recipient sites; and implanting harvested hair follicles.
Venus Legacy™ is cleared by the FDA for the non-invasive treatment of moderate to severe facial wrinkles and rhytides in females with Fitzpatrick skin types I-IV with the OctiPolar™ and DiamondPolar™ applicators, and temporary reduction in the appearance of cellulite with the 4D Body (LB2) and 4D Face (LF2) applicators. It is licensed by Health Canada and has CE Mark for the temporary increase of skin tightening, temporary circumferential reduction, temporary cellulite reduction, and temporary wrinkle reduction.
Venus Velocity™ is cleared by the FDA, licensed by Health Canada and has CE Mark for hair removal, permanent hair reduction (defined as the long-term stable reduction in the number of hairs re-growing when measured at 6, 9 and 12 months after the completion of a treatment regimen), and the treatment of pseudofolliculitis barbae for all Fitzpatrick skin types.
Venus Fiore™ received regulatory approval in Israel for aesthetic and functional treatment of the vagina, labia and mons pubis. Venus Fiore™ is available for sale in India, Hong Kong, and other selected Asian countries.
Venus Viva™ is cleared by the FDA, licensed by Health Canada, and has CE Mark for dermatological procedures requiring ablation and resurfacing of the skin. The DiamondPolar™ applicator is cleared by the FDA, licensed by Health Canada and has CE Mark for the treatment of moderate to severe wrinkles and rhytides in Fitzpatrick skin types I-IV.
Venus Freeze Plus™ is cleared by the FDA for the non-invasive treatment of moderate to severe facial wrinkles and rhytides in females with Fitzpatrick skin types I-IV. It is licensed by Health Canada for temporary skin tightening, and temporary reduction in the appearance of cellulite on the abdomen and flanks, using the DiamondPolar™ and OctiPolar™ applicators. The DiamondPolar™ applicator on Venus Freeze Plus™ has CE Mark for the non-invasive treatment of moderate to severe facial wrinkles and rhytides, and the increase of skin tightening, temporary circumferential reduction, and cellulite reduction with the OctiPolar™ applicator.
Venus Heal™ is licensed by Health Canada and can be used for the treatment of both acute and chronic disorders of the musculoskeletal system, such as muscle spasms, back pain, and soft tissue injuries, and results in effects such as pain relief, myorelaxation, increase of local blood circulation, and edema reduction. In the U.S., Venus Heal™ is cleared by the FDA for the relief of minor muscle aches and pain, relief of muscle spasm, and temporary improvement of local blood circulation. These indications enable the treatment of certain soft tissue injuries and conditions.
Venus Glow™ is cleared by the FDA as a Class I motorized dermabrasion device. It provides a dermal rejuvenation treatment that works to open up and deep-clean pores. Venus Concept is the exclusive distributor for Venus Glow™.
Venus Epileve™ is licensed by Health Canada and has CE Mark for hair removal, permanent hair reduction (defined as the long-term stable reduction in the number of hairs re-growing when measured at 6, 9 and 12 months after the completion of a treatment regimen), and the treatment of pseudofolliculitis barbae for all Fitzpatrick skin types. Venus Epileve™ is also CE-Marked for hirsutism.
Venus Freeze™ is cleared by the FDA for the non-invasive treatment of moderate to severe facial wrinkles and rhytides in females with Fitzpatrick skin types I-IV. It is licensed by Health Canada for temporary skin tightening, and temporary reduction in the appearance of cellulite on the abdomen and flanks, using the DiamondPolar™ and OctiPolar™ applicators. The DiamondPolar™ applicator on Venus Freeze™ has CE Mark for the non-invasive treatment of moderate to severe facial wrinkles and rhytides, and the increase of skin tightening, temporary circumferential reduction, and cellulite reduction with the OctiPolar™ applicator.
Venus Swan™ is cleared by the FDA for the non-invasive treatment of moderate to severe facial wrinkles and rhytides, and licensed by Health Canada for the non-invasive treatment of cellulite reduction, skin tightening, and temporary reduction in the appearance of stretch marks.
Copyright © 2025 Venus Concept. All rights reserved.
You are entering our website. For other countries/regions and language options, please click the SELECT A DIFFERENT REGION button below.
SELECT A DIFFERENT REGIONAre you looking to get a treatment? Please visit our patient website to learn more.
Click HereUnsure which aesthetic treatment is right for you? Take this quick and easy quiz to discover treatments that suit your needs.
Get Started