- Global
Get non-surgical solutions for today's top aesthetic concerns with Venus Treatments. Join thousands of satisfied patients worldwide!
- Loading...
- All Regions
Get non-surgical solutions for today's top aesthetic concerns with Venus Treatments. Join thousands of satisfied patients worldwide!
Diminish dark spots and other signs of premature aging for a more youthful, radiant complexion
Have you started noticing small brown spots on your skin? If so, you’re not alone. Many patients who have spent extended time in the sun are vulnerable to experiencing accelerated signs of premature aging on their face, hands and decollate. These sun spots are a form of skin damage, and they represent one of the most common skin concerns. Fortunately, there's a simple solution to improve your skin tone and restore a more youthful-looking complexion, a photofacial treatment.
Venus Concept's IPL treatment is a simple, non-surgical way to effectively target pigment under the skin's surface to reduce the appearance of discoloration and premature aging. There is minimal discomfort and typically only some redness for about a day afterward. With IPL photofacials, you can achieve noticeably brighter, clearer, and more youthful-looking skin. Each photorejuvenation treatment also works on vascular marks, such as spider veins and capillaries. All it takes is a few quick treatments, each of which lasts about 15 to 20 minutes and fits easily into your busy schedule or even on a lunch break.
Venus Concept's photofacial treatments are powered by Intense Pulsed Light (IPL) technology to safely and effectively fade common signs of premature aging, including sun spots, discoloration, and visible veins. IPL photofacial technology sends precise light through several layers of skin. This targets skin imperfections, correcting them without damaging any surrounding tissue.
Everything you need to know about the Venus Versa™ IPL photofacial treatment.
The best candidate is someone who has an uneven complexion with signs of discoloration or visible veins on their skin. IPL photofacials may also work to improve the look of fine lines.
Photofacial treatments work best for light to medium skin tones.
Absolutely. Exact treatment times may vary depending on the patient, but a single session typically takes 15-20 minutes.
Most patients receive 4-5 treatments per area. The exact number will depend on each individual person and the area being treated.
Treatments are done 3 weeks apart.
Make sure your skin is clean. Do not apply any lotions, creams, or makeup before your session, and stop using any products that might irritate your skin 2-3 days before. You must also avoid tanning, including with self-tanners. You may be advised to shave the area before your treatment. Remove all jewelry around the area being treated.
One session usually lasts 15-20 minutes, depending on the area being treated.
The IPL treatment feels like a light snap or flick on the surface of the skin.
The applicator has a cooling feature built into it to make the treatment as comfortable as possible, but you might feel a bit of discomfort if you’re treating a more sensitive area, like the upper lip. If you find it too uncomfortable, let the operator know and they can adjust the treatment accordingly.
Your skin may feel warm, like a sunburn, and can remain red for anywhere from a few hours to a day. Be sure to follow instructions for before and after your treatment.
It really depends on how your skin reacts to the treatment and how you care for your skin afterward. For example, if you protect your skin from the sun, the results will last longer. We recommend one touch-up session every 3-6 months, depending on your skin responds to the treatment.
There is no downtime. You can return to your daily schedule immediately after your treatment.
Avoid tanning for at least 2 weeks before and 2 weeks after your treatment, and wear sunscreen. You should also avoid hot baths, massages, or any treatment that requires direct contact with the skin for the first 2 days after your treatment.
Yes, you can apply makeup immediately after your treatment, but make sure you put on sunscreen first.
Extraordinary results speak for themselves
Fade the appearance of discoloration on the skin’s surface for a more even-toned complexion.
Devices that offer photofacial: Venus Versa™
Results after: 1 treatment
Courtesy of Tal Nachlieli, MD
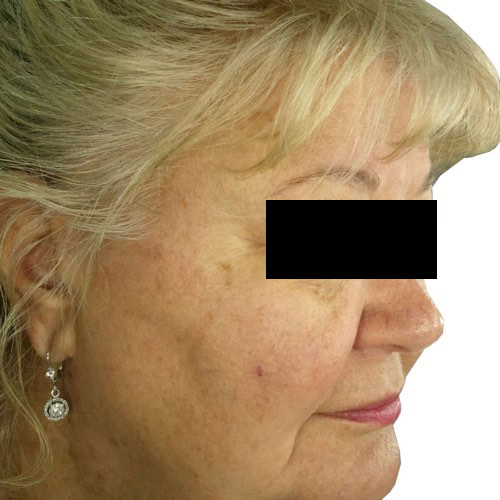 After
After
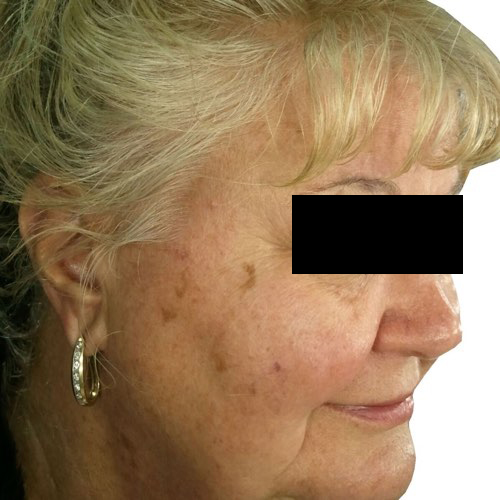 Before
BeforeFade the appearance of discoloration on the skin’s surface for a more even-toned complexion.
Devices that offer photofacial: Venus Versa™
Results after: 1 treatment
Courtesy of Tal Nachlieli, MD
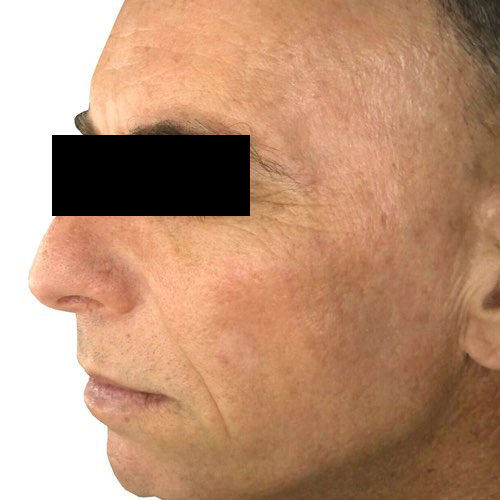 After
After
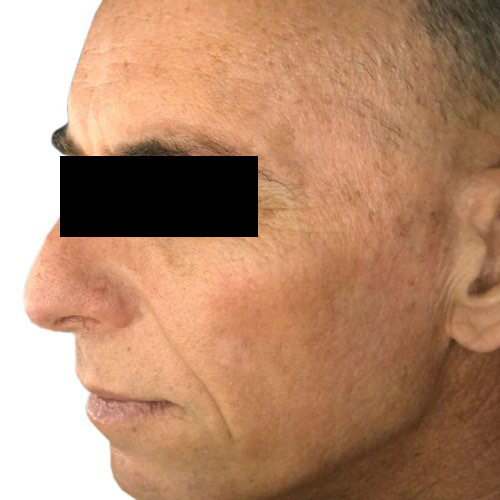 Before
BeforeINDICATIONS FOR USE:
Venus Versa™ is cleared by the FDA, licensed by Health Canada, and has CE Mark as a multi-application device intended to be used in aesthetic and cosmetic procedures. The SR515 and SR580 applicators are cleared by the FDA, licensed by Health Canada, and have CE Mark for the treatment of benign pigmented epidermal and cutaneous lesions and treatment of benign cutaneous vascular lesions. The HR650/HR650XL and HR690/HR690XL applicators are cleared by the FDA, licensed by Health Canada, and have CE Mark for the removal of unwanted hair and to effect stable long-term or permanent hair reduction for Fitzpatrick skin types I-IV. The AC Dual applicator is cleared by the FDA, licensed by Health Canada, and has CE Mark for the treatment of acne vulgaris. The DiamondPolar™ and OctiPolar™ applicators on the Venus Versa™ system are cleared by the FDA for non-invasive treatment of moderate to severe facial wrinkles and rhytides on females with Fitzpatrick skin types I-IV. The DiamondPolar™ applicator is licensed by Health Canada and has CE Mark for non-invasive treatment of moderate to severe facial wrinkles and rhytides on females with Fitzpatrick skin types I-IV. The OctiPolar™ applicator on the Venus Versa™ system is licensed by Health Canada and has CE Mark for temporary body contouring via skin tightening, circumferential reduction, and cellulite reduction. The NanoFractional RF™ (Viva) applicator is cleared by the FDA, licensed by Health Canada, and has CE Mark for dermatological procedures requiring ablation and resurfacing of the skin.
Search below to find a provider near you and to learn about our non-surgical aesthetic treatments with ARTAS®, NeoGraft®, Venus Bliss™, Venus Blilss MAX™, Venus Versa™, Venus Legacy™ Venus Versa™ Pro, Venus Velocity™, Venus Viva™ MD, and Venus Glow™.
For more information call: (888) 907-0115 // [email protected] // 235 Yorkland Blvd., Suite 900, Toronto, ON, M2J 4Y8 Canada
REGULATORY CLEARANCES [ More ]
Venus Bliss™ is cleared by the FDA and licensed by Health Canada for non-invasive lipolysis of the abdomen and flanks in individuals with a Body Mass Index (BMI) of 30 or less, with the diode laser applicators. The (MP)2 applicator is cleared by the FDA for temporary reduction in the appearance of cellulite, and licensed by Health Canada for temporary increase of skin tightening, temporary circumferential reduction, and temporary cellulite reduction. Venus Bliss™ has CE Mark as a non-invasive medical aesthetic device enabling a comprehensive approach leading to body contouring, addressing fat reduction, skin tightening, circumference reduction, and cellulite reduction.
Venus Versa™ is cleared by the FDA, licensed by Health Canada, and has CE Mark as a multi-application device intended to be used in aesthetic and cosmetic procedures. The SR515 and SR580 applicators are cleared by the FDA, licensed by Health Canada, and have CE Mark for the treatment of benign pigmented epidermal and cutaneous lesions and treatment of benign cutaneous vascular lesions. The HR650/HR650XL and HR690/HR690XL applicators are cleared by the FDA, licensed by Health Canada, and have CE Mark for the removal of unwanted hair and to effect stable long-term or permanent hair reduction for Fitzpatrick skin types I-IV. The AC Dual applicator is cleared by the FDA, licensed by Health Canada, and has CE Mark for the treatment of acne vulgaris. The DiamondPolar™ and OctiPolar™ applicators on the Venus Versa™ system are cleared by the FDA for non-invasive treatment of moderate to severe facial wrinkles and rhytides on females with Fitzpatrick skin types I-IV. The DiamondPolar™ applicator is licensed by Health Canada and has CE Mark for non-invasive treatment of moderate to severe facial wrinkles and rhytides on females with Fitzpatrick skin types I-IV. The OctiPolar™ applicator on the Venus Versa™ system is licensed by Health Canada and has CE Mark for temporary body contouring via skin tightening, circumferential reduction, and cellulite reduction. The NanoFractional RF™ (Viva) applicator is cleared by the FDA, licensed by Health Canada, and has CE Mark for dermatological procedures requiring ablation and resurfacing of the skin.
NeoGraft® is cleared by the FDA, licensed by Health Canada and has CE Mark with indication for use in suction-assisted follicular extraction and re-implantation. It is an auto-graft system and can be used on both male and female patients.
ARTAS iX™ is cleared by the FDA, licensed by Health Canada and has CE Mark with indication for use for harvesting hair follicles from the scalp in men diagnosed with androgenic alopecia (male pattern hair loss) who have black or brown straight hair. ARTAS iX™ is intended to assist physicians in identifying and extracting hair follicular units from the scalp during hair transplantation; creating recipient sites; and implanting harvested hair follicles.
Venus Legacy™ is cleared by the FDA for the non-invasive treatment of moderate to severe facial wrinkles and rhytides in females with Fitzpatrick skin types I-IV with the OctiPolar™ and DiamondPolar™ applicators, and temporary reduction in the appearance of cellulite with the 4D Body (LB2) and 4D Face (LF2) applicators. It is licensed by Health Canada and has CE Mark for the temporary increase of skin tightening, temporary circumferential reduction, temporary cellulite reduction, and temporary wrinkle reduction.
Venus Velocity™ is cleared by the FDA, licensed by Health Canada and has CE Mark for hair removal, permanent hair reduction (defined as the long-term stable reduction in the number of hairs re-growing when measured at 6, 9 and 12 months after the completion of a treatment regimen), and the treatment of pseudofolliculitis barbae for all Fitzpatrick skin types.
Venus Fiore™ received regulatory approval in Israel for aesthetic and functional treatment of the vagina, labia and mons pubis. Venus Fiore™ is available for sale in India, Hong Kong, and other selected Asian countries.
Venus Viva™ is cleared by the FDA, licensed by Health Canada, and has CE Mark for dermatological procedures requiring ablation and resurfacing of the skin. The DiamondPolar™ applicator is cleared by the FDA, licensed by Health Canada and has CE Mark for the treatment of moderate to severe wrinkles and rhytides in Fitzpatrick skin types I-IV.
Venus Freeze Plus™ is cleared by the FDA for the non-invasive treatment of moderate to severe facial wrinkles and rhytides in females with Fitzpatrick skin types I-IV. It is licensed by Health Canada for temporary skin tightening, and temporary reduction in the appearance of cellulite on the abdomen and flanks, using the DiamondPolar™ and OctiPolar™ applicators. The DiamondPolar™ applicator on Venus Freeze Plus™ has CE Mark for the non-invasive treatment of moderate to severe facial wrinkles and rhytides, and the increase of skin tightening, temporary circumferential reduction, and cellulite reduction with the OctiPolar™ applicator.
Venus Heal™ is licensed by Health Canada and can be used for the treatment of both acute and chronic disorders of the musculoskeletal system, such as muscle spasms, back pain, and soft tissue injuries, and results in effects such as pain relief, myorelaxation, increase of local blood circulation, and edema reduction. In the U.S., Venus Heal™ is cleared by the FDA for the relief of minor muscle aches and pain, relief of muscle spasm, and temporary improvement of local blood circulation. These indications enable the treatment of certain soft tissue injuries and conditions.
Venus Glow™ is cleared by the FDA as a Class I motorized dermabrasion device. It provides a dermal rejuvenation treatment that works to open up and deep-clean pores. Venus Concept is the exclusive distributor for Venus Glow™.
Venus Epileve™ is licensed by Health Canada and has CE Mark for hair removal, permanent hair reduction (defined as the long-term stable reduction in the number of hairs re-growing when measured at 6, 9 and 12 months after the completion of a treatment regimen), and the treatment of pseudofolliculitis barbae for all Fitzpatrick skin types. Venus Epileve™ is also CE-Marked for hirsutism.
Venus Freeze™ is cleared by the FDA for the non-invasive treatment of moderate to severe facial wrinkles and rhytides in females with Fitzpatrick skin types I-IV. It is licensed by Health Canada for temporary skin tightening, and temporary reduction in the appearance of cellulite on the abdomen and flanks, using the DiamondPolar™ and OctiPolar™ applicators. The DiamondPolar™ applicator on Venus Freeze™ has CE Mark for the non-invasive treatment of moderate to severe facial wrinkles and rhytides, and the increase of skin tightening, temporary circumferential reduction, and cellulite reduction with the OctiPolar™ applicator.
Venus Swan™ is cleared by the FDA for the non-invasive treatment of moderate to severe facial wrinkles and rhytides, and licensed by Health Canada for the non-invasive treatment of cellulite reduction, skin tightening, and temporary reduction in the appearance of stretch marks.
Copyright © 2025 Venus Concept. All rights reserved.
You are entering our website. For other countries/regions and language options, please click the SELECT A DIFFERENT REGION button below.
SELECT A DIFFERENT REGIONAre you looking to get a treatment? Please visit our patient website to learn more.
Click HereUnsure which aesthetic treatment is right for you? Take this quick and easy quiz to discover treatments that suit your needs.
Get Started